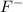Answer: The stable mono atomic ion of fluorine is
Step-by-step explanation:
Mono atomic ion is defined as the ion which is formed by 1 element only.
An ion is formed when an atom looses or gains electron.
- When an atom looses electrons, it will form a positive ion known as cation.
- When an atom gains electrons, it will form a negative ion known as anion.
Fluorine is the 9th element in the periodic table. The electronic configuration of this element is
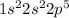
This element will gain 1 electron to form
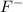 ion.
ion.
Hence, the stable mono atomic ion of fluorine is