Answer: The chemical formula for Palladium(IV) sulfate is
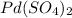
Step-by-step explanation:
Palladium is the 46th element of the periodic table which has an oxidation state of +4 (because it is a metal and will form positive ion) and sulfate is the polyatomic ion having a charge of -2 with formula
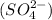
So, to write the formula of the compound, we do criss cross method in which oxidation state of each element or ion is given to the other combining atom which results into the formation of a neutral compound.
The chemical formula of the compound is
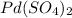
The image is given below.