Answer:
Number of carbon atoms present in the sample are 391.
Explanation:
We are given that,
Ratio of carbon to hydrogen atoms = 1 : 4
It is given that a sample of methane consists of 1564 hydrogen atoms.
Let the carbon atoms in the sample = x
So, we have the relation,
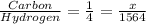
i.e.
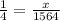
i.e.
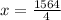
i.e. x= 391
Hence, the number of carbon atoms present in the sample are 391.