0.125 mg.
Step-by-step explanation
The mass of a radioactive species decrease by
 by the end of each half-life.
by the end of each half-life.
For this 1.00 mg sample of unknown half-life:
Mass of the sample at the end of the n-th half-life;
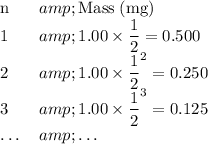 .
.
As a result,
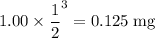
of the sample remains after three half-lives.