It takes 0.333 mol (33.7 g) of KNO₃ to make 21 g of HNO₃.
Step-by-step explanation
KNO₃ reacts with concentrated H₂SO₄ to produce HNO₃ and KHSO₄.
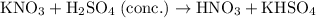 (balanced.)
(balanced.)
Each mole formula unit of KNO₃ reacts with excess H₂SO₄ to produce one mole of HNO₃. In other words, it takes the same number formula units of KNO₃ to produce a certain number of moles of HNO₃.
How many moles of molecules in 21 g of HNO₃?
Relative atomic mass:
- H: 1.008;
- N: 14.007;
- O: 15.999.
Molar mass of HNO₃:
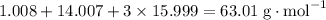 .
.
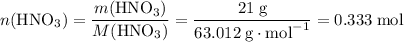 .
.
It takes 0.333 moles formula units of KNO₃ to produce 21 g or 0.333 moles of HNO₃.
What's the mass of 0.333 moles formula units of KNO₃?
Molar mass of KNO₃:
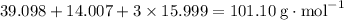
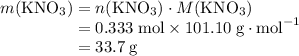 .
.