Answer: d. 22.4 L
Step-by-step explanation:
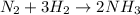
According to Avogadro's law , 1 mole of every gas occupies 22.4 L of volume at STP.
moles of ammonia=
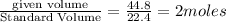
Given: Hydrogen is the excess reagent and nitrogen is the limiting reagent as it limits the formation of product.
From the given balanced equation:
2 moles of ammonia is produced by 1 mole of nitrogen.
Thus 2 moles of ammonia is produced by 22.4 L of nitrogen at STP as 1 mole of nitrogen occupies 22.4 L at STP.