Answer: Because more energy is required to form
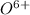 ion.
ion.
Step-by-step explanation:
Oxygen is the 8th element of the periodic table which belongs to Group 16.
The electronic configuration of the element is:
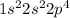
The number of valence electrons are 6 which is not a stable electronic configuration.
To attain stable electronic configuration, this element will either loose 6 electrons to form
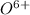 ion or gain 2 electrons to form
ion or gain 2 electrons to form
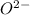 ion.
ion.
The loosing of 6 electrons require a huge amount of energy and is an unfavorable reaction. Hence, this element will gain 2 electrons easily and form
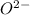 ion.
ion.
This element is considered as a non-metal because it gains electron to attain stable electronic configuration.