Answer: ethylene glycol (molar mass = 62.07 g/mol)
Explanation:
Depression in freezing point :
Formula used for lowering in freezing point is,
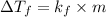
or,
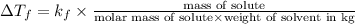
where,
 = change in freezing point
= change in freezing point
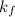 = freezing point constant=
= freezing point constant=
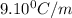
m = molality
Given mass of solute = 0.807 g
Molar mass of solute=? g/mol
weight of solvent in kg= 11.6 g=0.0116 kg
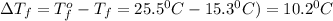
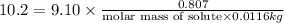
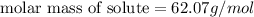
Thus the solute is ethylene glycol which has same molecular mass as calculated, i.e 62.07 g/mol.