The cell notation is:
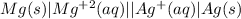
here in cell notation the left side represent the anodic half cell where right side represents the cathodic half cell
in anodic half cell : oxidation takes place [loss of electrons]
in cathodic half cell: reduction takes place [gain of electrons]
1) this is a galvanic cell
2) the standard potential of cell will be obtained by subtracting the standard reduction potential of anode from cathode
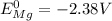
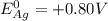
Therefore
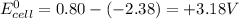
3) as the value of emf is positive the reaction will be spontaneous as the free energy change of reaction will be negative
Δ
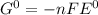
As reaction is spontaneous and there will be conversion of chemical energy to electrical energy it is a galvanic cell.