2.88 grams.
Step-by-step explanation
Al is in excess. 6.52 grams of Al converted to only 5.95 grams of Al₂(SO₄)₃. At least some Al atoms is in excess. In other words, Au₂SO₄ is the limiting reactant. All 3.58 grams of Au₂SO₄ is converted.
Refer to the conservation of atoms to avoid balancing the equation. Au atoms conserve. There are two Au atoms in one formula unit of Au₂SO₄. As a result, two moles of Au atoms will be produced when one mole formula units of Au₂SO₄ reacts completely with Al.
How many moles of formula units in 3.58 grams of Au₂SO₄?
Refer to a modern periodic table for relative atomic mass data:
- Au: 196.967;
- S: 32.06;
- O: 15.999.
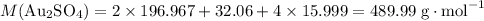 .
.
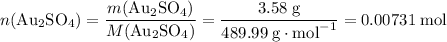 .
.
How many moles of Au atoms will be produced from 0.00731 mol of Au₂SO₄?
There are two Au atoms in each formula unit of Au₂SO₄. Both Au atoms will end up as Au (s) as Au₂SO₄ reacts with excess Al.
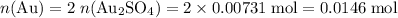 .
.
What's the mass of 0.0146 mol of Au?
The relative atomic mass of Au is 196.967.
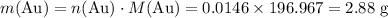 .
.