Answer:
Q = 3.33 108 J
Step-by-step explanation:
This is an exercise in thermodynamics, specifically isobaric work
W = V (
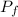 - P₀)
- P₀)
They tell us that we have ⅓ of the volume of the container filled with water
V = ⅓ 100
V = 33.3 m³
let's calculate
W = 33.3 (90-100) 10⁶
W = - 3.33 10⁸ J
To bring the system to its initial condition if we use the first law of thermodynamics
ΔE = Q + W
as we return to the initial condition the change of internal energy (ΔE) is zero
W = -Q
therefore the required heat is
Q = 3.33 108 J