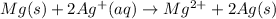Step-by-step explanation:
The given following standard cell notation.
Mg(s) | Mg^2+ (aq) || Aq^+(aq) | Aq(s)
Oxidation:
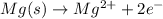 ....(1)
....(1)
Magnesium metal by loosing 2 electrons is getting converted into magnesium cation. Hence, getting oxidized
Reduction:
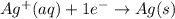 ...(2)
...(2)
Silver ion by gaining 1 electrons is getting converted into silver metal. Hence, getting reduced.
Overall redox reaction: (1)+2 × (2)