Answer : This is a type of decomposition reaction. The mass of copper oxide produced would be, 159 grams
Solution : Given,
Mass of copper sulfate = 319.22 g
Molar mass of copper sulfate = 159.6 g/mole
Molar mass of copper oxide = 79.5 g/mole
First we have to calculate the moles of copper sulfate.
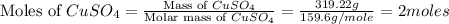
Now we have to calculate the moles of copper oxide.
The balanced chemical reaction is,
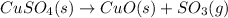
This reaction is a decomposition reaction in which the larger reactant molecule decomposes to give two or more new products.
From the reaction, we conclude that
As, 1 mole of copper sulfate decomposes to produce 1 mole of copper oxide
So, 2 moles of copper sulfate decomposes to produce 2 moles of copper oxide
Now we have to calculate the mass of copper oxide.
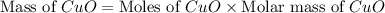
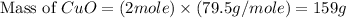
Therefore, the mass of copper oxide produced would be, 159 grams