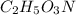Answer: The molecular formula of the compound is
Step-by-step explanation:
To know the molecular formula of the compound, we will follow some steps:
Step 1: Converting all these percentages into mass.
We take the total mass of the compound to be 100 grams, so, the percentages given for each element becomes its mass.
Mass of Carbon =
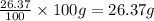
Mass of Hydrogen =
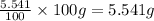
Mass of Oxygen =
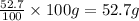
Mass of Nitrogen =
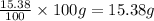
Molar mass of Carbon = 12 g/mole
Molar mass of Hydrogen = 1 g/mole
Molar mass of Oxygen = 16 g/mole
Molar mass of Nitrogen = 14 g/mol
Step 2: Converting the given masses into their respective moles.
Moles of Carbon =

Moles of Hydrogen =
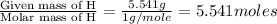
Moles of Oxygen =
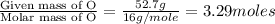
Moles of Nitrogen =
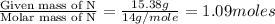
Step 3: Now, calculating mole ratio, we divide each number of moles by the smallest number of moles calculated.
For Carbon =
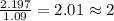
For Hydrogen =
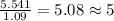
For Oxygen =
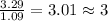
For Nitrogen =
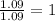
The ratio of C : H : O : N = 2 : 5 : 3 : 1
Step 4: Writing the mole ratio of the element as the subscripts in molecular formula.
Molecular formula of the compound :
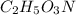
Hence, the molecular formula of the compound is