Answer: 13 grams
Explanation:
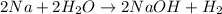
According to avogadro's law, 1 mole of every substance occupies 22.4 Liters at STP and contains avogadro's number
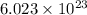 of particles.
of particles.
According to stoichiometry,
1 mole of
 is produced from 2 moles of sodium
is produced from 2 moles of sodium
or 22.4 L of
 at STP is produced from =2 moles of sodium
at STP is produced from =2 moles of sodium
thus 6.30 L of
 at STP is produced from =
at STP is produced from =
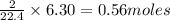 of sodium
of sodium
To calculate the mass of sodium metal used
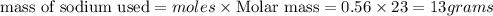
Thus the initial quantity of sodium metal used is 13 grams.