1 g requires 80 cal of heat, then 100 g requires 80*100=8000 cal
1 cal = 4.1868 J
Then Q = 1910.767 J
for you:
The heat energy is defined from the equation:
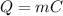 Δ
Δ

where
- Q: the heat energy in (Joules).
- m: the mass of the material which is heated in (kg).
- C: the specific heat capacity in (J/kg.K).
- Δ
 : the change in the temperature in (K)
: the change in the temperature in (K)