Answer: Amount of sodium chloride produced is 25.43 grams.
Step-by-step explanation:
To calculate the moles:
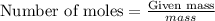
Given: Mass of sodium = 10 g
Molar mass of sodium = 23g/mol
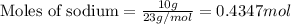
The balanced equation is:
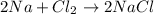
By Stoichiometry of the reaction,
2 moles of sodium produces 2 moles of sodium chloride
So, 0.4347 moles of sodium will produce =
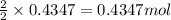 of sodium chloride.
of sodium chloride.
Molar mass of sodium chloride = 58.5 g/mol
Putting values in equation 1, we get:
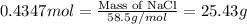
Hence, amount of sodium chloride produced is 25.43 grams.