Answer: The equilibrium constant for the given reaction is 0.0421.
Step-by-step explanation:
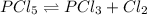
Concentration of
![[PCl_5]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/rc87xg5qaqryj7zehjf9z7nzrowpz4bmfb.png) = 0.0095 M
= 0.0095 M
Concentration of
![[PCl_3]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/itcwuwtju5b44v6qedg8mo0ar2o2th8d4i.png) = 0.020 M
= 0.020 M
Concentration of
![[Cl_2]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/v75bkiprjls8kiamt8nz609kyhnons8726.png) = 0.020 M
= 0.020 M
The expression of the equilibrium constant is given as:
![K_c=([PCl_3][Cl_2])/([PCl_5])=(0.020 M* 0.020 M)/(0.0095 M)](https://img.qammunity.org/2020/formulas/chemistry/middle-school/94tbvlx5nnwef2y592m8ckrz2jsnd7ij3r.png)
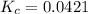 (An equilibrium constant is an unit less constant)
(An equilibrium constant is an unit less constant)
The equilibrium constant for the given reaction is 0.0421.