Answer: Amount of iron produced is 101.36 grams.
Step-by-step explanation:
For the reaction of aluminium and iron oxide, the equation follows:
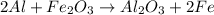
By Stoichiometry of the reaction,
When 1 mole of aluminium oxide is produced, then 2 moles of iron is also produced.
So, when 0.905 moles of aluminium oxide is produced, then
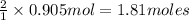 of iron is also produced.
of iron is also produced.
Now, to calculate the amount of iron produced, we use the equation:

Molar mass of iron = 56 g/mol
Moles of iron = 1.81 moles
Putting values in above equation, we get:
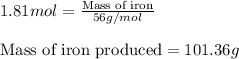
Hence, amount of iron produced is 101.36 grams.