Answer: Oxygen
Step-by-step explanation:
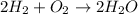
As seen from the balanced equation:
2 moles of hydrogen combine with 1 mole of oxygen or 1 mole of hydrogen combine with 1/2 mole of oxygen.
According to Avogadro's law, equal volumes of gases contain equal number of molecules and hence equal number of moles.
Thus if x moles of both gases are present, x moles of hydrogen will combine with
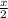 moles of oxygen and
moles of oxygen and
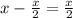 moles of oxygen will be left used and will be in excess.
moles of oxygen will be left used and will be in excess.