Answer: 938.203 grams of zinc metal will react completely with 8.2 liters of 3.5 M HCl.
Step-by-step explanation:
Molarity of the HCl solution = 3.5 M = 3.5 mol/L
Volume of the HCl solution = 8.2 l
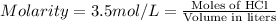
Moles of HCl =
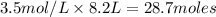
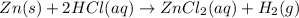
According to reaction 2 moles of the HCl reacts with 1 mole of Zn metal.
Then 28.7 moles of HCl will react with:
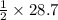 moles of Zinc metal that is 14.35 moles.
moles of Zinc metal that is 14.35 moles.
Mass of the zinc metal required to completely react with given HCl solution is:
Mass of Zinc = Moles of zinc × Atomic Mass of zinc
= 14.35 mol × 65.38 g/mol = 938.203 g
938.203 grams of zinc metal will react completely with 8.2 liters of 3.5 M HCl.