Answer: The amount of heat released is 84 calories.
Explanation:
The equation used to calculate the amount of heat released or absorbed, we use the equation:
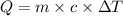
where,
Q = heat gained or released = ? Cal
m = mass of the substance = 10g
c = specific heat of aluminium = 0.21 Cal/g ° C
Putting values in above equation, we get:
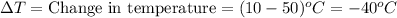
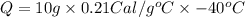
Q = -84 Calories
Hence, the amount of heat released is 84 calories.