Answer : The resulting temperature of gas will be, 580 K
Explanation :
Charles' Law : It is defined as the volume of gas is directly proportional to the temperature of the gas at constant pressure and number of moles.
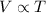
or,
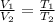
where,
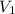 = initial volume of gas = 40.0 mL
= initial volume of gas = 40.0 mL
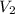 = final volume of gas = 80.0 mL
= final volume of gas = 80.0 mL
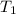 = initial temperature of gas =
= initial temperature of gas =
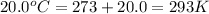
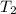 = final temperature of gas = ?
= final temperature of gas = ?
Now put all the given values in the above formula, we get the final temperature of the gas.
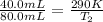
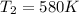
Therefore, the resulting temperature of gas will be, 580 K