Names ought to be:
a. Iron(II) bromide.
b. Carbon disulfide.
c. Cobalt(III) nitrate.
d. Magnesium hydroxide.
e. Copper(I) oxide.
See the explanation for why the names given are not appropriate.
Step-by-step explanation
Metals and nonmetals tend to form ionic compounds when the two combine. Metals may also combine with polyatomic ions like
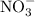 to produce an ionic compound. a, c, d, and e are all ionic compounds. Names for ionic compounds should not include numerals such as "di-" or "tri-". Also, indicate the oxidation state for transition elements using Roman numerals in brackets.
to produce an ionic compound. a, c, d, and e are all ionic compounds. Names for ionic compounds should not include numerals such as "di-" or "tri-". Also, indicate the oxidation state for transition elements using Roman numerals in brackets.
The name in a is not correct since:
- Iron Fe is a transition element. Its oxidation state is missing.
- There's a prefix in front of "bromide" despite FeBr₂ is an ionic compound.
The name in b is not correct since:
- C stands for carbon. Cu is the symbol for copper.
- CS₂ is a covalent compound between two nonmetals. The prefix that indicates the number of sulfur atoms in the molecule is missing.
The name in c is not correct since:
- Cobalt Co is a transition element. Its oxidation state is missing.
- There's a prefix in front of "nitrate" despite Co(NO₃)₂ is an ionic compound.
The name in d is not correct since:
- There's a prefix in front of "hydroxide" despite Mg(OH)₂ is an ionic compound.
The name in e is not correct:
- There's a prefix in front of "copper" despite Cu₂O is an ionic compound.
- The oxidation state for copper in Cu₂O should be +1 rather than +2.