Answer:
1.91
Explanation:
The chlorous acid is 340 000 times stronger than the hypochlorous acid. It will suppress the ionization of HOCl to such an extent that we can ignore the HOCl in our calculations.
The equation for the equilibrium of HClO₂ is
HClO₂ + H₂O ⇌ H₃O⁺ + ClO₂⁻; Kₐ= 1.2 × 10⁻²
For ease of typing, let’s rewrite the equation as
HA + H₂O ⇌ H₃O⁺ + A⁻
Set up an ICE table:
HA + H₂O ⇌ H₃O⁺ + A⁻
I: 0.025 0 0
C: -x +x +x
E: 0.025-x x x
Set up the Kₐ expression
Kₐ = {[H₃O⁺][A⁻]}/[HA] = 1.2 × 10⁻² Substitute values
(x × x)/(0.0250-x) = 1.2 × 10⁻² Combine like terms
x²/(0.025-x) = 1.2 × 10⁻²
Check that x ≪ 0.025
0.025/(1.2 × 10⁻²) = 2.1
The ratio is less than 400. We must solve a quadratic equation
Set up the quadratic equation.
x²/(0.025-x) = 1.2 × 10⁻²
x² = (0.025 - x)(1.2 × 10⁻²)
x² = 3.00 × 10⁻⁴ - 1.2 × 10⁻²x
x² + 1.2×10⁻²x – 3.00×10⁻⁴ = 0
Solve the quadratic equation
The solutions to the quadratic equation are
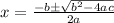
a = 1
b = 1.1 × 10⁻²
c = -1.65 × 10⁻³
Substituting the values into the equation, we get
x = 0.0123 and x = -0.02435
b =We reject the negative value, so
x = [H₃O⁺] = 0.0123 mol·L⁻¹
Calculate the pH
pH = -log[H₃O⁺]
pH = -log(0.0123)
pH = 1.91