Answer: The formula for the given compound is C2H2Br2Cl2.
Step-by-step explanation:
We are provided with a compound in the image and we need to find the molecular formula for the compound.
The compound given has 2 atoms of carbon (C), 2 atoms of hydrogen (H), 2 atoms of bromine (Br) and 2 atoms of chlorine (Cl).
Hence, we first write the symbol of these elements and the number of atoms are written as subscripts.
So, the formula for the given compound is
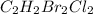 which is written as C2H2Br2Cl2.
which is written as C2H2Br2Cl2.