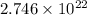Answer: The number of C-atoms in methylacetylene are
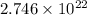
Step-by-step explanation:
To calculate the number of atoms, we first need to calculate the number of moles. For that we use the equation of ideal gas, which is:

where,
P = pressure of the gas = 1 atm
V = Volume of the gas =
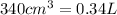 (Conversion Factor:
(Conversion Factor:
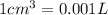 )
)
n = Number of moles = ?
R = gas constant =
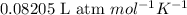
T = temperature of the gas =
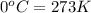 (Conversion factor:
(Conversion factor:
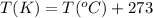 )
)
Putting values in above equation, we get:
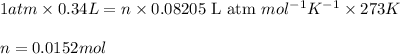
According to the mole concept:
1 mole of a compound contains
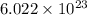 number of atoms
number of atoms
As, 1 mole of methylacetylene contains
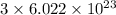 number of C-atoms.
number of C-atoms.
So, 0.0152 moles of methylacetylene contains
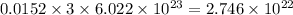 number of C-atoms.
number of C-atoms.
Hence, the number of C-atoms in methylacetylene are