Answer: 37.9 mL
Density is the ratio between the mass and volume of a substance, or between the mass of a substance and the mass of an equal volume of another substance taken as a standard. If we want to know what is the volume that represents a certain mass of a liquid we can use density as a conversion factor.
To know the volume (V) of ethanol required for the preparation of the drug we must divide the mass (m) of ethanol between the density of this liquid:
d =
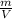 → V =
→ V =
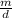
→ V =
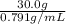
→ V = 37.9 mL
The volume of ethanol required to prepared the medication 37.9 mL