Hello!
The answer is: 4.71 grams of Hydrogen
Why?
From the statement, we know that for the studied compound, which has a mass of 124 g, we have that 3.80% is Hydrogen.
So, to find how many grams of Hydrogen is in 124 grams of the compound, we need to do the same calculation:
First, we need to convert the 3.80% percent in a real number, we can do it by dividing the percent into 100:
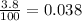
Then,
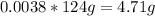
So, there are 4.71 grams of Hydrogen in 124 grams of the compound.
Have a nice day!