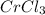We know that
1) empirical formula of a compound is the representation of a molecules with the elements in simplest mole ratio.
2) the molar mass is the actual proportion of moles of elements present in a molecule
3) there is a simple ratio between the molar mass and empirical mass a molecule
Now let us solve each problem
1) empirical formula of
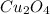 will be
will be
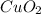
2) empirical formula of
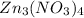 will be
will be
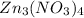
3) empirical formula of
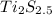 will be
will be
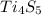
4) the molecular formula will be :
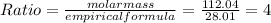
the molecular formula = 4 X empirical formula = 4 X CO =
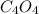
5) the mole ratio of Cl and Cr is
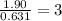
empirical formula will be