Answer: 245 feet
Explanation:
Given: The height of the rocket after t seconds is represented by the formula:
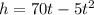
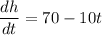 [Differentiate both sides w.r.t. t]
[Differentiate both sides w.r.t. t]
Put
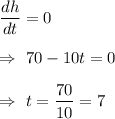
Also,
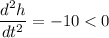 i.e. height is maximum at t=7.
i.e. height is maximum at t=7.
Maximum height =
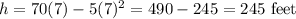
Hence, the maximum height the rocket reaches = 245 feet.