Step-by-step explanation:
Le Chatelier's principle states that for a long period of time if a system is at equilibrium and it is subjected to change in concentration, temperature, volume or pressure then the system shifts to a new equilibrium.
This change will partly counter acts the applied change.
Therefore, when heat is added to the system then equilibrium will shift to the side where temperature or heat is reduced again.
For example,
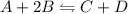
Since heat is added to the system, hence, system will shift to the left side or we can say equilibrium will shift to the backward direction.