Answer: Option d) 37 g
Solution:
This question can be solved using the Graham's Law which states that:
Rate of effusion or diffusion of gas is indirectly proportional to the square root of its Molar Mass.
For two gases A and B, this formula can be written as:
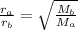
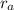 = Rate of effusion of gas A
= Rate of effusion of gas A
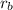 = Rate of effusion of gas B
= Rate of effusion of gas B
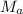 = Molar mass of gas A
= Molar mass of gas A
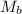 = Molar mass of gas B
= Molar mass of gas B
We are given that, Gas A effuses 0.68 times as fast as Gas B. This means:
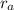 = 0.68 x
= 0.68 x
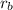
Using these values in the formula of Graham's law, we get:
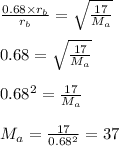
Therefore, mass of gas A is 37 g, rounded to nearest unit.