Answer: 1.61 x 10⁴ kPa
Dalton's law states that the sum of the partial pressures of each gas equals the total pressure of the gas mixture. According to this law,
Pi = xi P
where Pi is the partial pressure of the gas i, xi is the mole fraction of the gas i in the gas mixture and P is the total pressure.
The mole fraction is defined as the quotient between the moles of solute (ni) and the total moles of the mixture (nt), which is calculated by adding the moles of all its components:
xi =
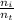
In the Trimix 10/50 mix you have 10% oxygen, 50% helium and 40% nitrogen.
To calculate the total number of moles of the mixture and thus determine the molar fraction of helium, we consider 100 g and calculate the number of moles that represent 10 g of O₂ (n₁), 50 g of He (n₂) and 40 g of N₂ (n₃):
n₁ = 10 g x
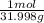 = 0.313 mol
= 0.313 mol
n₂ = 50 g x
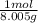 = 6.246 mol
= 6.246 mol
n₃ = 40 g x
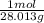 = 1.428 mol
= 1.428 mol
Then the total number of moles (nt) will be:
nt = n₁ + n₂ + n₃ = 0.313 mol + 6.246 mol +1.428 mol
nt = 7,987 mol
Then, the mole fraction of helium (x₂) in the mixture will be,
x₂ =
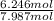 = 0.78
= 0.78
and the partial pressure of helium in the mixture, according to Dalton's law, will be:
P₂ = x₂ P = 0.78 x 2.07 x 10⁴ kPa
P₂= 1.61 x 10⁴ kPa
So, the partial pressure of helium if a tank of trimix 10/50 has a total pressure of 2.07 x 104 kPa is 1.61 x 10⁴ kPa