Answer: 100 grams of the parent isotope will remain after one half life.
Step-by-step explanation:
Mass of the isotope present at initial stage =
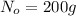
The mass of the parent isotope left after the time ,t=N
Time taken by the samle ,t =
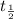
The half life of the sample :
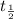
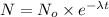
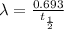
![\ln[N]=ln[N^o]-\frac{0.693}{t_{(1)/(2)}}* t_{(1)/(2)}](https://img.qammunity.org/2020/formulas/biology/middle-school/4t1nvgld288j1ntqf5p7tgwsj7uu21lnt6.png)
![2=([N_o])/([N])](https://img.qammunity.org/2020/formulas/biology/middle-school/2gjrqdnh09pywf2xacx2mthmf10vb2ui2u.png)
![[N]=(N_o)/(2)=(200 g)/(2)=100 g](https://img.qammunity.org/2020/formulas/biology/middle-school/4shv8y1mvbo1zsq7o23fvinvercm3373d5.png)
100 grams of the parent isotope will remain after one half life.