Answer: 4 moles of
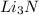
Step-by-step explanation:
The balanced chemical equation for the reaction is:
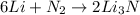
As can be seen from the chemical equation, 6 moles of lithium reacts with 1 mole of nitrogen to produce 2 moles of lithium nitride.
Given : Lithium is the limiting reagent as it limits the formation of product and nitrogen is the excess reagent as it is present in excess.
Now if 6 moles of lithium produces = 2 moles of
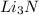
Thus 12 moles of lithium produces =
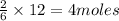 of
of
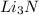
Thus 4 moles of
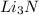 will be produced.
will be produced.