Answer: This element has 3 valance electrons and it will loose electrons to form a
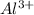 ion.
ion.
Step-by-step explanation:
Aluminium is the 13th element in our periodic table.
The valance shell electronic configuration of this element is:
![[Ne]3s^23p^1](https://img.qammunity.org/2020/formulas/chemistry/middle-school/uud3u094nik5stg8236eujfz16gt2mxb3w.png)
As, there are 3 electrons in the outermost shell, Therefore, total number of valence electrons of this element are 3.
For this element loosing 3 electrons will be easy than to gain 5 electrons to acquire a full outer most shell configuration. Therefore, this element will loose 3 electrons.
When this element looses 3 electrons, it looses negative charge and starts to form positive ion. And it form an ion having +3 charge.