Hello!
If I initially have a gas at a pressure of 12 atm, volume of 23 liters, and temperature of 200 K, and then I raise the pressure to 14 atm and increase the temperature to 300 K, what is the new volume of the gas?
We have the following data:
P1 (initial pressure) = 12 atm
V1 (initial volume) = 23 L
T1 (initial temperature) = 200 K
P2 (final pressure) = 14 atm
T2 (final temperature) = 300 K
V2 (final volume) = ? (in L)
Now, we apply the data of the variables above to the General Equation of Gases, let's see:
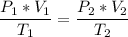
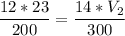
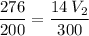
multiply the means by the extremes
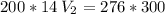
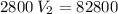
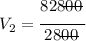
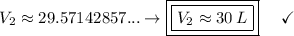
*** Note: the approximation rule says that when the number before the digit 5 is odd, the previous value is raised to the next even number
Answer:
The new volume is approximately 30 Liters
_______________________