Answer:
if kinetic energy is doubled than temperature of the gas must be double
Step-by-step explanation:
As we know that average kinetic energy of the gas atom is known as
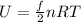
here we know that
f = degrees of freedom
R = ideal gas constant
T = temperature of the gas
now we know that average kinetic energy of the gas is double that of initial value
so from above formula we can say that kinetic energy is directly proportional to the temperature of the gas
so here if kinetic energy is doubled than temperature of the gas must be double