Answer : The final energy of the system if the initial energy was 2000 J is, 3500 J
Solution :
(1) The equation used is,
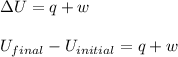
where,
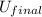 = final internal energy
= final internal energy
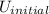 = initial internal energy
= initial internal energy
q = heat energy
w = work done
(2) The known variables are, q, w and
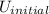
initial internal energy =
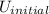 = 2000 J
= 2000 J
heat energy = q = 1000 J
work done = w = 500 J
(3) Now plug the numbers into the equation, we get
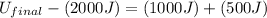
(4) By solving the terms, we get
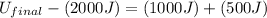
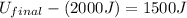
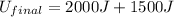
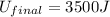
(5) Therefore, the final energy of the system if the initial energy was 2000 J is, 3500 J