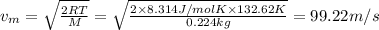Step-by-step explanation:
Let the temperature at which gas is moving be T
Pressure exerted by the gas , P = 4.35 atm
Volume occupied by the gas ,V = 3.5 l
Moles of bromine gas,n = 1.4 moles
Consider the bromine gas is behaving ideal .

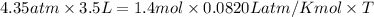
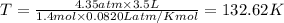
Mass of bromine gas,M = Moles × Molar mass = 1.4 mole × 160 g/mol = 224 g = 0.224 kg
Value of R = 8.314 J/mol K
Root mean square velocity of the bromine gas:
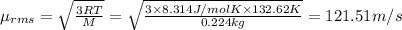
Average velocity of the bromine gas:
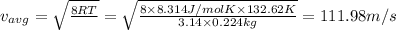
Most probable velocity: