Answer : The correct option is, 11.77 L
Solution : Given,
Mass of bromine gas = 84 g
Molar mass of bromine gas = 159.8 g/mole
First we have to calculate the moles of bromine gas.
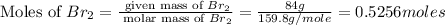
Now we have to calculate the volume of bromine gas at STP.
As we know that at STP,
1 mole of bromine gas contains 22.4 L volume of bromine gas
So, 0.5256 mole of bromine gas contains
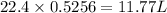 volume of bromine gas
volume of bromine gas
Therefore, the volume of bromine gas at STP is, 11.77 L