Answer:
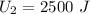
Explanation:
To solve this problem, we must use the first law of thermodynamics that says that the net heat absorbed by a system is equal to the sum of the work done plus the change of internal energy.
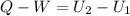
*
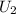 = final internal energy of the system
= final internal energy of the system
*
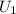 = initial internal energy of the system
= initial internal energy of the system
*
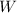 = Work done by the system
= Work done by the system
*
 = Heat added to the system
= Heat added to the system
Then:
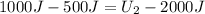
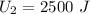
The final internal energy of the system is 2500 J. This means that by adding 1000j of heat to the system the internal energy increased by 500J