Answer: 47.04 L will be the volume occupied by the 2.1 moles of nitrogen gas at STP.
Step-by-step explanation:
At STP,
1 mol of ideal gas occupies = 22.4 L
Considering nitrogen gas to be an ideal gas
Then 2.1 moles of the nitrogen gas will occupy:
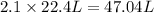
47.04 L will be the volume occupied by the 2.1 moles of nitrogen gas at STP.