Answer:- A precipitate of
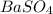 is formed and the spectator ions are
is formed and the spectator ions are
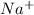 and
and
 .
.
Explanations:- A double replacement reaction takes place. In double replacement reaction, the ion exchange takes place and we write the products. Then we use the solubility chart to check if a precipitate forms.
The balanced molecular equation is:
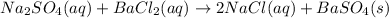
From solubility chart barium sulfate is insoluble so its precipitate is formed. sodium and chlorine ions are spectator ions as shown in the below ionic equation.
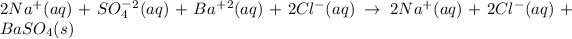
The net ionic equation is:
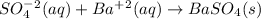
So, the spectator ions are
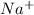 and
and
 and a precipitate of
and a precipitate of
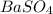 is formed.
is formed.