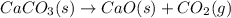Answer : The correct example of a decomposition reaction is, (A)
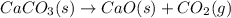
Explanation :
Decomposition reaction : It is defined as the reaction in which the the larger molecule decomposes to give two or more smaller molecules.
(A) The given balanced chemical reaction is,
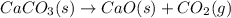
This reaction is a decomposition reaction in which the the larger molecule calcium carbonate decomposes to give two or more smaller molecules as calcium oxide and carbon dioxide.
(B) The given balanced chemical reaction is,
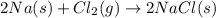
This reaction is a synthesis reaction where multiple substances or reactants combine to form a single product.
(C) The given balanced chemical reaction is,
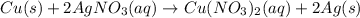
This reaction is a synthesis reaction in which the more reactive element replace the less reactive element.
(D) The given balanced chemical reaction is,
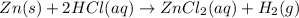
This reaction is a synthesis reaction in which the more reactive element replace the less reactive element.
Hence, the correct example of a decomposition reaction is, (A)