Answer:
3.5
Explanation:
Given : the hydronium ion concentration is
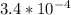 in orange juice
in orange juice
To Find: what is the pH?
Solution :
Definition of pH : pH is the negative log of the molar hydrogen ion concentration
Formula of pH : - log [H+]
Since we are given H+ =
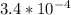
So, substituting this value in formula :
![pH = - log [3.4*10^(-4)]](https://img.qammunity.org/2020/formulas/mathematics/high-school/s6g8317ax9yg9zmfuqx4dwdcfy2x0ekg94.png)
![pH = - log [3.4*0.0001]](https://img.qammunity.org/2020/formulas/mathematics/high-school/kyfpcqteb5r8nh905jf0632q9darqufdm2.png)
![pH = - log [0.00034]](https://img.qammunity.org/2020/formulas/mathematics/high-school/f7uvt2ye6cs4lyjz7dm9bit08p1x8i14rb.png)
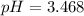
pH = 3.468≈3.5
Thus the pH is 3.5