Answer: 27.09 ppm and 0.003 %.
First, for air pollutants, ppm refers to parts of steam or gas per million parts of contaminated air, which can be expressed as cm³ / m³. Therefore, we must find the volume of CO that represents 35 mg of this gas at a temperature of -30 ° C and a pressure of 0.92 atm.
Note: we consider 35 mg since this is the acceptable hourly average concentration of CO per cubic meter m³ of contaminated air established in the "National Ambient Air Quality Objectives". The volume of these 35 mg of gas will change according to the atmospheric conditions in which they are.
So, according to the law of ideal gases,
PV = nRT
where P, V, n and T are the pressure, volume, moles and temperature of the gas in question while R is the constant gas (0.082057 atm L / mol K)
The moles of CO will be,
n = 35 mg x
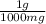 x
x
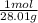
→ n = 0.00125 mol
We clear V from the equation and substitute P = 0.92 atm and
T = -30 ° C + 273.15 K = 243.15 K
V =
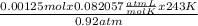
→ V = 0.0271 L
As 1000 cm³ = 1 L then,
V = 0.0271 L x
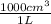 = 27.09 cm³
= 27.09 cm³
Then the acceptable concentration c of CO in ppm is,
c = 27 cm³ / m³ = 27 ppm
To express this concentration in percent by volume we must consider that 1 000 000 cm³ = 1 m³ to convert 27.09 cm³ in m³ and multiply the result by 100%:
c = 27.09
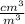 x
x
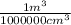 x 100%
x 100%
c = 0.003 %
So, the acceptable concentration of CO if the temperature is -30 °C and pressure is 0.92 atm in ppm and as a percent by volume is 27.09 ppm and 0.003 %.