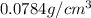Answer:The density of iron in
Step-by-step explanation:
The density of the crystal solids is calculated by using :
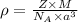
Z = number of atoms in crystal lattice in body centered cubic cell = 2
M = Atomic mass = 55.85 g/mol
a = Edge length of the crystal = 287 pm =
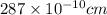
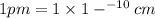
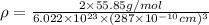
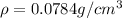
The density of iron in