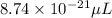Answer : The volume of a copper atom in micro-liters is,
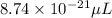
Solution : Given,
Volume of a single copper atom =
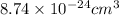
Now we have to calculate the volume of a copper atom in micro-liters.
The conversion used for this is,
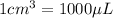
As, volume of a single copper atom in
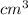 =
=
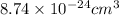
So, volume of a single copper atom in micro-liters =
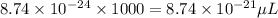
Hence, the volume of a copper atom in micro-liters is,